As we enter the second half of 2024, digital marketing continues to evolve at a breakneck pace. The way businesses connect with their audience is undergoing significant transformations, making a robust digital marketing strategy more crucial than ever.
A well-crafted digital marketing strategy is a must for businesses aiming to succeed in the modern marketplace. In this comprehensive exploration, we’ll delve into why a well-crafted digital marketing strategy is indispensable in 2024 and beyond.
Digital Marketing Strategy: The Game Changer
The digital revolution has transformed how businesses operate, interact with customers, and market their products. According to a report by Statista, global digital advertising spending is projected to reach $870.85 billion by 2027, up from $549.51 billion in 2022. This exponential growth underscores the shift towards digital platforms as the primary means of reaching and engaging with audiences.
A digital marketing strategy is a comprehensive plan that outlines how a business will leverage online channels to achieve its marketing objectives. It encompasses various elements, including content marketing, social media marketing, search engine optimization (SEO), email marketing, and pay-per-click (PPC) advertising. The goal is to create a cohesive approach that maximizes online presence, drives traffic, and converts leads into customers.
The Customer Journey in a Digital Marketing Strategy
The customer journey can be divided into the following stages: Awareness, Consideration, Decision, and Loyalty.
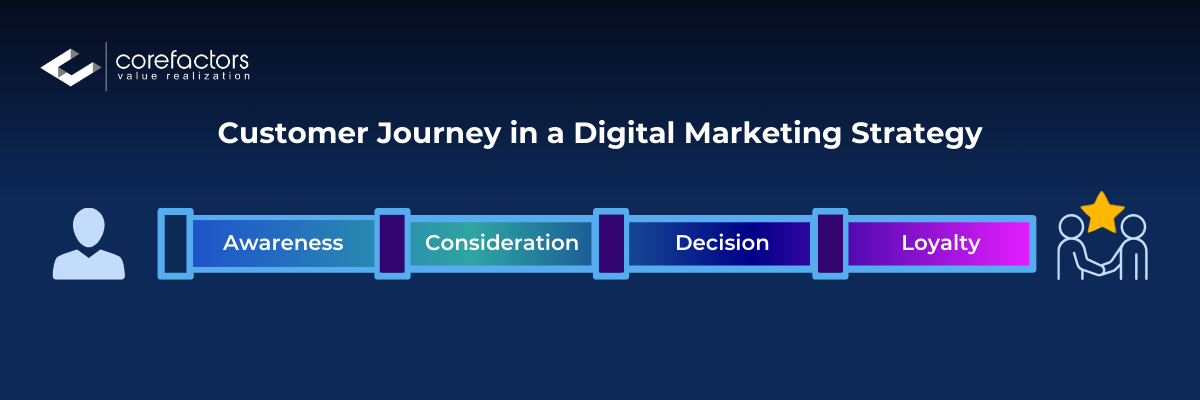
- Awareness: The customer becomes aware of the brand
Touchpoints: Social media ads, SEO, blog posts, online videos
Impact: High engagement rates and increased website traffic
2. Consideration: The customer evaluates the brand's offerings
Touchpoints: Email marketing, retargeting ads, influencer reviews, webinars
Impact: Higher click-through rates and growing interest in products/services
3. Decision: The customer decides to purchase
Touchpoints: Product pages, customer reviews, comparison charts, special offers
Impact: Increased conversion rates and higher sales volume
4. Loyalty: The customer becomes a repeat buyer and brand advocate
Touchpoints: Loyalty programs, personalized emails, social media engagement, customer support
Impact: Higher customer retention, positive word-of-mouth, increased lifetime value
Digital Marketing Trends in 2024
To stay ahead in the competitive market, businesses must keep abreast of the latest digital marketing trends. Here are some key trends that are shaping the digital marketing landscape in 2024.
- Social Media Marketing
- Short-Form Video Dominance: Platforms like TikTok, Instagram Reels, and YouTube Shorts have a growing rate of customer engagement.
- Augmented Reality (AR) Integration: Enhancing shopping experiences with virtual try-ons.
- Influencer Marketing Evolution: Rise of micro and nano influencers for higher engagement and authenticity.
2. Search Engine Optimization (SEO)
- Voice Search Optimization: Adapting content for natural language and conversational keywords due to the rise of smart speakers.
- Google's Search Generative Experience (SGE): AI-driven search providing more relevant and comprehensive information.
- High EAT Content: Emphasis on Experience, Expertise, Authoritativeness, and Trustworthiness in content.
- Focus on User Experience (UX): Enhancing page speed, mobile-friendliness, and overall UX for better rankings.
3. Artificial Intelligence and Machine Learning
- Personalization at Scale: Using AI to deliver tailored marketing campaigns based on customer data.
- Chatbots and Conversational AI: Improving customer service with AI-driven chatbots.
- Predictive Analytics: Utilizing AI and ML to forecast consumer behavior and make data-driven decisions.
- Content Creation and Curation: AI tools generate high-quality content and automate marketing briefs and social media posts.
4. Pay-Per-Click (PPC)
- AI in Google Ads: Enhanced ad creation, targeting, and optimization with AI integration.
- Increased Competition and Costs: Rising PPC costs due to higher competition, necessitate optimized ad spend.
- Ad Customization and Personalization: Leveraging AI for precise audience segmentation and personalized ad content.
- Automation Tools: Using automation for bid management, budget allocation, and performance tracking.
Why is Digital Marketing Important for the Future?
Let’s have a look at why digital marketing will become indispensable in the future.
1. Adapting to Rapid Technological Advancements
Technology is the backbone of digital marketing. With advancements in artificial intelligence (AI), machine learning (ML), and automation, businesses can reach their target audience with unprecedented precision.
According to a report by MarketsandMarkets, AI in the marketing market is projected to grow from USD 5.00 billion in 2017 to USD 40.9 billion by 2025, at a Compound Annual Growth Rate (CAGR) of 29.79%.
AI-powered tools enable personalized marketing campaigns, predictive analytics, and efficient customer service. A comprehensive digital marketing strategy leverages these technologies to stay ahead of the competition and meet the ever-changing demands of consumers.
2. Customer Experience is All That Matters
The way businesses make their customers feel can make or break their business. Today’s consumers expect seamless interactions and personalized experiences across all touchpoints.
A digital marketing strategy that prioritizes user experience can significantly enhance customer satisfaction and loyalty. This includes optimizing websites for mobile devices, creating engaging content, and utilizing chatbots for instant customer support. By focusing on the customer journey, businesses can build strong, lasting relationships.
3. Data-Driven Decision Making
One of the biggest advantages of digital marketing is the ability to collect and analyze data. In 2024 and beyond, data-driven decision-making is not just an option, it's a necessity.
A strategic approach to digital marketing involves leveraging data analytics to gain insights into consumer behavior, preferences, and trends. This allows businesses to make informed decisions, refine their marketing efforts, and achieve better ROI. The ability to track and measure the effectiveness of campaigns in real time is a game-changer.
Some examples can be,
- Scheduling Posts During Peak Hours - Schedule posts when your audience is most active to maximize engagement.
- Real-Time Analytics - Track metrics like click-through and conversion rates in real-time to adjust campaigns on the fly.
- Ad Performance Optimization - Quickly adjust content and visuals for underperforming ads based on real-time data.
- Personalized Email Content - Use data to tailor email content and product recommendations to individual preferences.
- A/B Testing - Test different website elements and email designs to find the most effective versions.
- Pricing Strategy - Adjust prices using insights from competitive analysis and customer purchasing behavior.
- SEO Optimization - Analyze search data to improve website content and achieve better search engine rankings.
- Sentiment Analysis - Analyze customer reviews and feedback to improve products and services.
- Budget Allocation - Allocate marketing budgets based on performance data across various channels and campaigns.
4. Increased Reach and Visibility
Now the internet is everywhere and so the interconnected network offers unparalleled reach. A well-executed digital marketing strategy ensures businesses can tap into new markets and reach a global audience. Social media platforms, search engine optimization (SEO), and pay-per-click (PPC) advertising are just a few channels that can amplify a brand's visibility. By diversifying their online presence, companies can attract a broader audience and drive more traffic to their websites.
5. Cost-Effectiveness
Compared to traditional marketing methods, digital marketing is often more cost-effective. It allows businesses to allocate their budgets more efficiently and precisely target specific demographics.
Digital strategies offer flexible and scalable options for businesses of all sizes, whether it’s through social media ads, email marketing, or content marketing. This cost-efficiency is especially beneficial for startups and small businesses looking to maximize their marketing budgets.
6. Building Brand Authority
In a crowded marketplace, establishing brand authority is vital. A strategic digital marketing approach helps businesses position themselves as industry leaders. This can be achieved through thought leadership content, engaging social media presence, and consistent brand messaging. By providing valuable information and insights, businesses can build trust and credibility with their audience, setting themselves apart from competitors.
7. Adapting to Consumer Behavior
Consumer behavior is constantly evolving, influenced by technological advancements and cultural shifts. In 2024, staying attuned to these changes is more important than ever.
A dynamic digital marketing strategy allows businesses to adapt quickly to new trends and consumer preferences. This agility is crucial for staying relevant and maintaining a competitive edge. By continuously monitoring and analyzing consumer behavior, businesses can tailor their marketing efforts to meet the current needs and desires of their audience.
Notable Trend: The rise of voice search is reshaping how consumers interact with content. It is predicted that more than 50% of all searches will be voice searches. Businesses need to optimize their content for voice search to capture this growing segment.
Futureproof Your Business Now
As we look ahead, the potential for growth and innovation in the digital marketing sphere is limitless. Businesses that are proactive, agile, and strategic in their approach will not only survive but thrive in this ever-evolving landscape. The business landscape is rapidly transforming, driven by technological advancements and evolving consumer behaviors.
Futureproof your business by investing in a dynamic and comprehensive digital marketing strategy and you’ll not only navigate the future—you'll lead it.

Frequently Asked Questions (FAQs)
1. Why is digital marketing important in 2024?
Digital marketing is crucial due to the rapid technological advancements and changing consumer behaviors, making it essential for businesses to connect with their audience effectively.
2. How can I use data analytics in my marketing strategy?
Data analytics can help track campaign performance, understand consumer behavior, optimize content, and make informed decisions to improve ROI.
3. What is the role of social media in digital marketing?
Social media platforms are vital for engaging with audiences, building brand awareness, and driving traffic to your website through targeted campaigns.
4. What is A/B testing, and why is it important?
A/B testing involves comparing two versions of a webpage or email to see which performs better, helping optimize designs and messaging for better results.
5. What is the future of digital marketing?
The future of digital marketing lies in embracing AI and machine learning, leveraging data for personalized experiences, and staying agile to adapt to new consumer trends and technologies.








