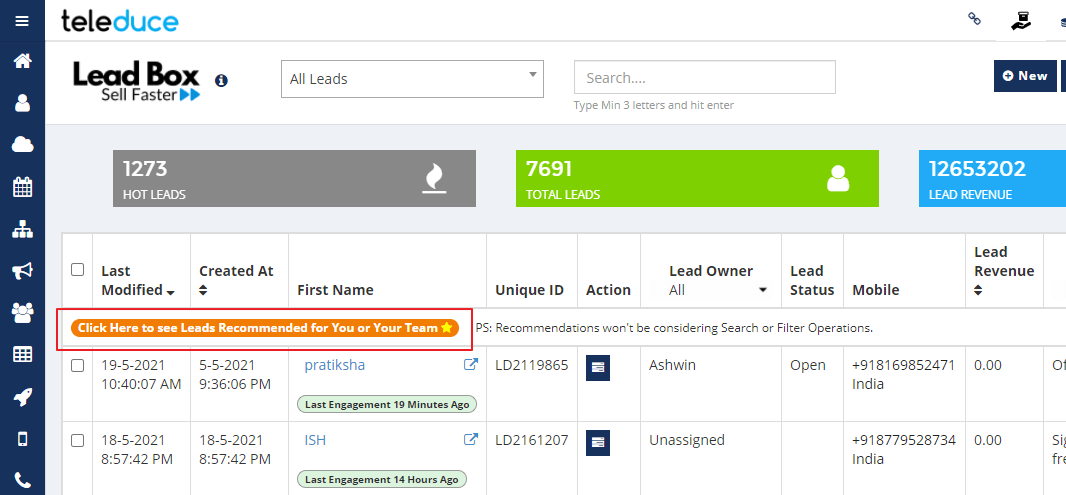
If you are stuck between a mountain of leads, this new feature is going to be really helpful for you. The recommended leads option is now available in Teleduce.
Recommended Leads helps you to quickly view:
- Leads having Pending Tasks Today
- Leads responded to Emails in Last 24 Hrs
- Marked as Hot Leads without any Recent Activities
We are sure that it will further help our users to reduce lead leakage and effectively and timely follow-up.
There was also an improvement in the UI of the Homepage. Now there’s an easier option to close tasks on the home page and inside the lead details page.
Also, now you can access the zip file as an attachment in Teleduce and save it. It would help you to store large file related to your leads.